How A CMMS Can Help Medical Device Manufacturers Prove Compliance
Read more about how CMMS captures signatures and audit trails to enhance compliance.
Solutions
Workplace Management Solutions
Real Estate Management Solutions
Maintenance Management Solutions
Energy Management Solutions
Engineering Document Management Solutions
Asset Management Solutions
Automate campus scheduling for classes, meetings, and exams with our EMS software.
Plan and manage conferences effortlessly with EMS software to impress guests and streamline operations.
Boost workplace flexibility and maximize space use with seamless desk and room booking.
Organize workplace or campus events smoothly, creating memorable experiences.
Optimize workspace, manage allocations efficiently, and reduce costs with our space management solutions.
Deliver projects on time and within budget by improving communication, collaboration, and efficiency with our software.
Streamline lease accounting for ASC 842, IFRS, and GASB compliance.
Manage leases efficiently by tracking key dates, analyzing costs, and ensuring compliance.
Centralize data and analytics for better insights, faster negotiations, and revenue growth.
Centralize facility and asset maintenance, automate work orders, and ensure compliance with our CMMS software.
Extend asset life, reduce downtime, and prevent costly repairs with data-driven monitoring.
Prevent equipment failures and extend asset life by detecting and addressing issues early.
Make sustainable, cost-efficient energy decisions by monitoring and optimizing power consumption.
Remotely monitor and control equipment with real-time data to predict issues, boost efficiency, and reduce downtime.
Easily share and collaborate on documents, creating a single source of truth for engineers and contractors.
Manage and analyze assets across their lifecycle to schedule maintenance, reduce downtime, and extend lifespan.
Improve visibility, automate work orders, and ensure compliance for efficient facility and asset management.
Resources
Browse our full library of resources all in one place, including webinars, whitepapers, podcast episodes, and more.
Self-Service & Support
Looking for self‑service training, best practices, helpful videos, product resources, or support? You’re in the right place.
About Accruent
Get the latest information on Accruent, our solutions, events, and the company at large.

Here's everything you need to know about FDA and CFR regulations including how the right tech can simplify your compliance efforts.
Many industries fall under the ‘Life Sciences’ umbrella: biotechnology, pharmaceuticals, healthcare technologies, biomedical devices, environmental sciences – the list goes on. And all these organizations have two things in common: first, as life science companies, they’re dedicated to researching and improving organism life. Second, they all have to deal with a lot of red tape and federal regulations to remain operational.
The second point is not glamorous, but it’s important – and compliance is becoming increasingly complex as factors like globalization, heightened transparency expectations, increased emphasis on innovation, and ever-evolving customer needs make regulations that much more complicated (and important).
In this context, many organizations are now finding their approach to compliance to be woefully lacking, and it can be hobbling their chance at ongoing success. After all, the more adeptly organizations can understand and adhere to the regulations that apply to them, the more effectively they can lower costs, streamline operations and focus on do their jobs, (i.e., making lives better).
Here’s everything you need to know about FDA regulations and how to simplify FDA compliance for organizations in life sciences.

The most important FDA regulations for organizations in the Life Science industries fall under the Title 21 CFR umbrella.
The U.S Food and Drug Administration (FDA), the Office on National Drug Control Policy (ONDCP) and the Drug Enforcement Agency (DEA) all use Title 21 Code of Federal Relations (CFR) to govern food, drugs, cosmetics and other public health products. When it comes to enforcement, inspections can be conducted for the purpose of pre-qualification, routine inspection, or “for-cause” to investigate a specific problem reported to the FDA – so you need to be consistently ready.
Title 21 CFR Part 11, in particular, is all about electronic signatures, electronic records, and ensuring that both components are trustworthy, reliable and equivalent to paper records (ERES). Generally speaking, part 11 applies to medical device manufacturers, drug makers, biotech companies, and other FDA-regulated industries.
This focus on electronic records may seem oddly specific, but establishing a trustworthy, consistent electronic paper trail is a major time-saver and necessary for maintaining record standards in a modern age. Before part 11 was established in 1997, institutions had to submit physical documents to be audited, and it cost a lot of time, physical space and efficiency. Part 11 makes it much easier to prove compliance and to comply with other regulatory sections.
That said, it’s still difficult to get right. Having electronic signatures and records that are up to regulatory standards requires audits, system validations, electronic signature software, and systems that can help store, organize, and process the electronic data that must be maintained.
Similarly, cGMP, or Current Good Manufacturing Practice, is a system enforced by the FDA to ensure that products are consistently produced and controlled according to quality standards. CGMPs assure proper design, monitoring and control of manufacturing processes and facilities.
You will see specific cGMP regulations for various product categories in Title 21 CFR, including:
This isn’t technically a regulation, but it’s important to mention in this context. GAMP®, or Good Automated Manufacturing Practice, is defined as a system for producing quality equipment using the concept of prospective validation following a life cycle model. In simpler terms, GAMP is a set of guidelines – for both users and manufacturers of pharmaceutical products – that helps pharmaceutical organizations understand how they should validate their computer systems to remain compliant with ever-changing FDA regulations.
GAMP is not a regulation in and of itself, but most pharmaceutical companies that want their automated systems to meet the FDA quality standards adopt GAMP principles and procedures to do so. The latest GAMP standard, GAMP-5, is the most structured and project-based approach to GAMP. It revolves around four tenets:
It’s a lot of moving parts – and FDA compliance is only one piece of a complex puzzle of regulations that organizations in life sciences must adhere to – but it’s important to get right.
It’s simple: if you want to run a sustainable, revenue-driven business, compliance is non-negotiable:
Unfortunately, the fact that compliance is so important doesn’t make it any easier to achieve.
With so many governments and agencies in the ring, compliance itself is a complicated and wild ecosystem – and many life science organizations don’t have a comprehensive understanding of the landscape or where their organization stands.
Usually, life science companies tend to use historical data for compliance – but real-time data and analytics could help organizations more effectively identify and address incoming risks.
Many companies are doing everything they can to deliver new technologies that address unmet patient needs. That said, there’s a lot of confusion when it comes to new tech and compliance, and companies often have poor communication with regulators. Translation? Major compliance complications down the road.
In many organizations, various teams – including quality, clinical, manufacturing, regulatory and IT teams – work without collaborating with one another.
Most organizations don’t have a centralized repository of compliance resources that their employees can consult – and this is a huge misstep. What’s more, important documentation to obtain compliance and meet audit requirements isn’t usually widely available or comprehensive, making it difficult for regulated companies to show proof of compliance when regulators arrive.
Oftentimes, organizations are operating using a patchwork of modern and legacy systems and processes. This:
It’s also becoming explicitly frowned upon by the FDA, which is starting to expect automation in compliance — particularly with things like electronic medical-device reporting (eMDR) and electronic common technical document (eCTD).
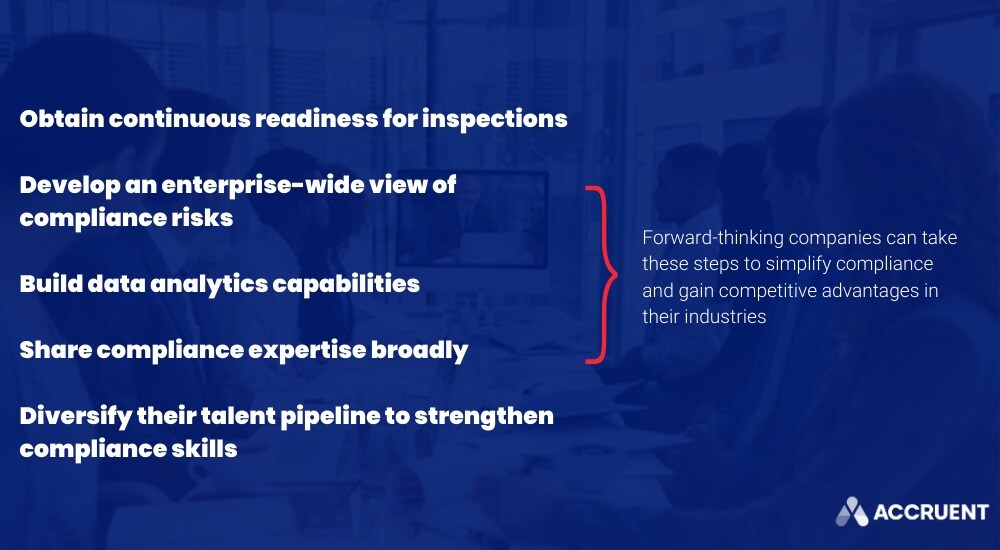
The right technological ecosystem, culture and business structure can address many of these concerns and simplify compliance, helping you support your team, facilitate effective processes and turn your compliance policies from a cost into a sustainable competitive advantage (even when a new product launches or a new business relationship is explored).
This, according to Deloitte, requires that organizations:
These points, in turn, require a well-connected, robust and user-friendly technological ecosystem.
The right computerized maintenance management system software (CMMS), for example, can strengthen data integrity and help significantly with the FDA validation process (while leading to improved efficiencies, decreased downtime, elevated employee satisfaction and higher ROI).
Similarly, a robust cloud-based electronic document management system can:
Read more about how CMMS captures signatures and audit trails to enhance compliance.
See how this cloud-based, pre-validated engineering document management system (EDMS) can solve your greatest document, validation, and compliance ...
To comply with FDA Title 21 CFR Part 11 regulations, follow these seven helpful tips, including how to choose a pre-validated document software ...
Subscribe to stay up to date with our latest news, resources and best practices.
* To unsubscribe at any time, please use the “Unsubscribe” link included in the footer of our emails.